Tumors are frequently hypoxic, and glycolytic. Their metabolic pathways are rerouted to help tumor cells continue to divide rapidly under lack of oxygen, and unavailability of certain nutrients. We study how lactate, a byproduct of anaerobic glycolysis, and accompanied acidosis in the microenvironment influences cancer phenotypes.
We have also discovered that HIF-1 activity can fluctuate, driven by lactate, allowing cells to cheat and grow. Using temporal control of gas exchange, we study how fluctuations in hypoxia can specifically modulate cancer behaviors.
Key Findings
- Hypoxia Inducible Factor-1 is the key transcriptional regulator of hypoxic response in metazoans. When oxygen is low, HIF-1 alpha continuous O2 dependent degradation is inhibited, leading to HIF-1 accumulation. We found that even in hypoxia, subpopulations of cancer cells can emerge which fluctuate HIF-1 transcriptional activity, providing an opportunity to cheat and continue to divide. Crucially, this is because of lactate, which accumulates in glycolytic tumors and can promote chaperone mediated autophagy of HIF-1. These cells could act as feeder cells for tumor growth (Kshitiz et al., Cell Systems, 2022).
- Using microscopy, dynamic O2 control, multi-omics, and computational approaches, we have shown that unstable hypoxia can result increased cell growth, pro-metastatic CAF activation, and lower patient survival (Suhail Y., Molecular Carcinogenesis, 2024).

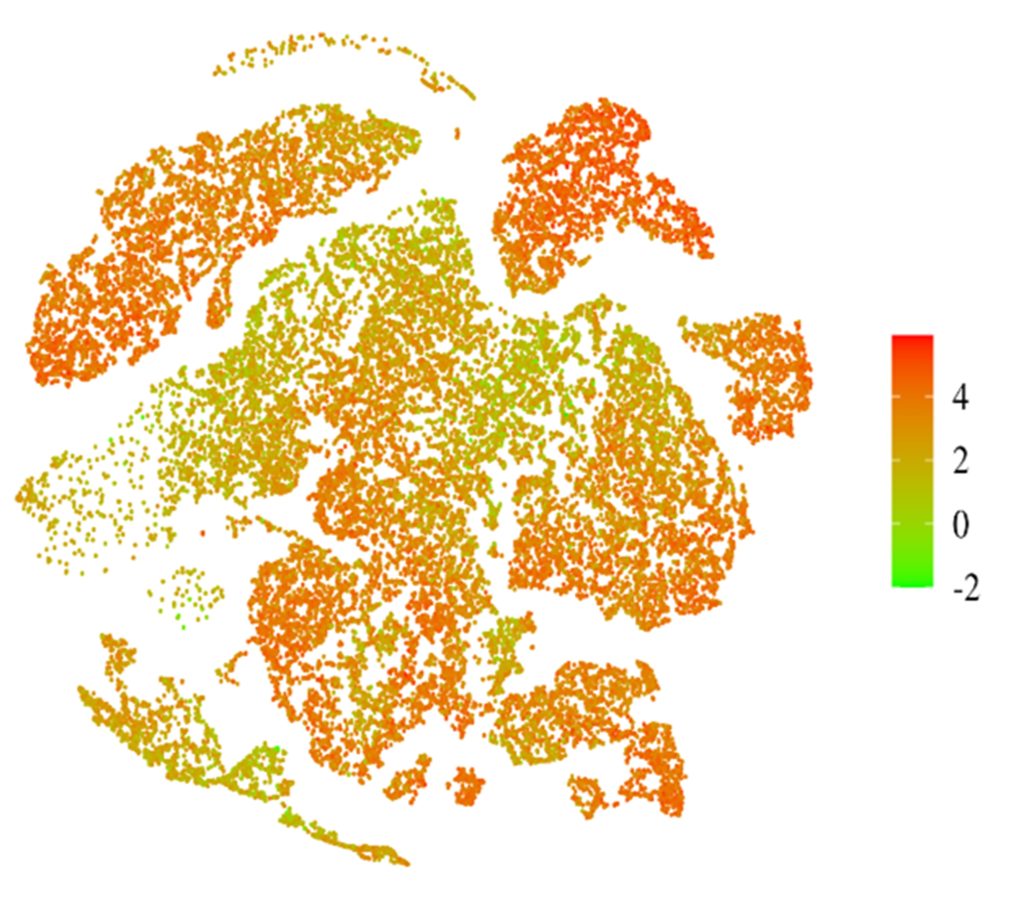
- Different tissues experience different ambient O2 levels normally. Lung, for example, is highly oxygenated, while pancreas have a low O2 tension at homeostasis. Does this affect the cancers in these different environments, and particularly the response of HIF-1 induced transcription? Computing a HIF-1 z-score to predict downstream gene expression in scRNAseq data, we have found that cells in pancreatic, lung, and colon cancers respond very differently to HIF-1 activation at the gene expression level (Liu, S. et al., Molecular Carcinogenesis, 2024).
Relevant Publications
- Suhail Y, Liu Y, Du W, Afzal J, Qiu X, Atiq A, Vera-Licona P, Agmon, E, Kshitiz (2024), Oscillatory hypoxia induced gene expression predicts low survival in human breast cancer patients, Molecular Carcinogenesis.
- Liu Y, Suhail Y, Novin A, Afzal J, Pant A, Kshitiz (2024), Lactate in breast cancer cells is associated with evasion of hypoxia-induced cell cycle arrest and adverse patient outcome, Human Cell 37, 768-781.
- Liu S, Liu Y, Qiu X, Suhail Y, Kshitiz (2024), Tissue-of-origin for cancers determine HIF-1 activation induced phenotypic heterogeneity, Molecular Carcinogenesis.
- Novin A, Wali K, Pant A, Liu S, Du W, Liu Y, Wang L, Xu M, Wang B, Suhail Y, Kshitiz (2024), Oscillatory Hypoxia can Induce Senescence of Adipose-derived Mesenchymal Stromal Cells Potentiating Invasive Transformation of Breast Epithelial Cells, Cancers 16(5), 969.
- Du W, Novin A, Liu Y, Afzal J, Liu S, Suhail Y, Kshitiz (2024), Oscillatory hypoxia induced gene expression predicts low survival in human breast cancer patients, Mechanobiology in Medicine 3, 100070-11.
- Kshitiz±, Afzal J, Suhail Y, Chang H, Hubbi ME, Hamidzadeh A, Goyal R, Liu Y, Sun P, Dang CV±, Levchenko A± (2022), Lactate-dependent chaperone-mediated autophagy induces oscillatory HIF-1a activity promoting proliferation of hypoxic cells, Cell Systems, Accepted. ± Corresponding authors.
- Gassaway BM, Cardone RL, Padyana AK, Petersen MC, Judd ET, Hayes S, Tong S, Barber KW, Apostolidi M, Abulizi A, Sheetz JB, Kshitiz, Aerni HR, Gross S, Kung C, Samuel VT, Shulman GI, Kibbey RG, Rinehart J (2019). Distinct Hepatic PKA and CDK Signaling Pathways Control Activity-Independent Pyruvate Kinase Phosphorylation and Hepatic Glucose Production, Cell Reports, 29(11), 3394-3404.
- Ahn EH, Lee MB, SeoDJ, Lee J, Kim Y, Gupta K (2018). Sphigosine Induced Apoptosis and Down-regulation of MYCN in PAX3-FOXO1-positive Alveolar Rabdomyocarcoma Cells Irrespective of TP53 Mutation, Anticancer Research, 38(1), 71-76.
- Hubbi ME, Gilkes DM, Hu H, Kshitiz, Ahmed I, Semenza GL (2014). Cyclin-dependent kinases regulate lysosomal degradation of hypoxia-inducible factor 1-alpha to promote cell-cycle progression, Proceedings of the National Academy of Sciences, E3325-3334.
- Hubbi ME, Hu H, Kshitiz, Gilkes DM, Semenza GL (2013). Sirtuin-7 inhibits the activity of hypoxia-inducible factors, J. Biol. Chem., 288(29):20768-75.
- Hubbi ME, Hu Hongxia, Kshitiz, Ahmed I, Levchenko A, Semenza GL (2013). Chaperone-mediated autophagy targets HIF1a for lysosomal degradation, J. Biol. Chem., 288(15):10703-14.
- Chaturvedi P, Gilkes DM, Wong CCL, Kshitiz, Luo W, Zhang H, Wei Hn Takano N, Schito L, Levchenko A, Semenza GL (2012), Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis, The Journal of Clinical Investigation, 123(1).
- Rey S, Lee K, Wang CJ, Gupta K, Chen S, McMillan A, Bhise N, Levchenko A, Semenza GL. (2009). Synergistic effect of HIF-1alpha gene therapy and HIF-1-activated bone marrow-derived angiogenic cells in a mouse model of limb ischemia, Proc Natl Acad Sci USA. 106(48):20399-404.
