A very long collaboration that started as a side gig in my postdoc with Chi V Dang (at UPenn), and continued over a decade through my stint at Yale with Andre Levchenko, and then for years at UConn is finally out! It all started with the observation of a strange phenomenon while I was characterizing a vector Chi V Dang’s lab had created. We found that a small number of cancer cells were not maintaining their HIF transcriptional activity at stable levels, and were cheating the other cells in hypoxia. There is much more to this story, so read along: (here is the link).
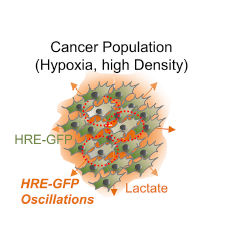
The observation was made a while back, but it took a long time to fully work out how the cells actually cheated, and whether it mattered in real cancers. Under hypoxia, cells stabilize a protein called HIF-1, which is a master regulator of oxygen response in the cells. When oxygen goes down, for example, HIF-1 signaling becomes high and takes the cells to an entirely new state. HIF-1 directs the cell division machinery to stop working, jumpstarts anaerobic respiration using a large quantity of glucose, and also makes cells secrete proteins to bring blood vessels towards themselves. We noted that noted that a small percentage of cells did not stabilize HIF-1, but instead oscillated it. As HIF-1 oscillated, and went from up to down to up again, cells could escape the HIF-1 imposed pause and continue to divide. In this way, these oscillating cells cheated and continue to divide, in spite of the very low oxygen levels.
We also found that cancer cells were communicating with each other, through lactate, a byproduct of glycolysis which is increased in hypoxia. At that time, lactate (or other metabolites) were not considered as signaling molecules, so it was quite a surprise. Cancers can accumulate a lot of lactate in their environment. We found was that it was lactate which was destabilizing HIF-1 in a small number of cells, pointing to a new role of lactate as a messenger. With Junaid Afzal (then at Hopkins), we worked out the details of the mechanism, finding that lactate was inducing increased degradation of HIF-1 in the lysosomes. HIF-1 is classically degraded in proteosomes, but a while back my friend, Maimon Hubbi from Semenza lab had reported (in a paper I co-authored) that HIF-1 could be degraded in lysosomes too, but we really did not know much significance of this finding. So it is satisfying that degradation of HIF-1 in lysosomes could allow a dynamic control of its activity.
Eventually at UConn, Yasir Suhail worked out the significance of these findings. He , looked at these genes in all human cancers and found a surprising presence of these genes in most cancers. Genes which are turned off by oscillating hypoxia were turned off in most cancers. It appeared that oscillation in HIF-1 levels may decrease tumor suppressor genes, and contribute to cancer growth in most cancers. The most interesting aspect is the universality of the phenomenon in all cancers. It seems this effect is pan-cancer, and not just in any cancer.
This unique phenomenon answers many conundrums about cancer, while opening new lines of scientific enquiry.

